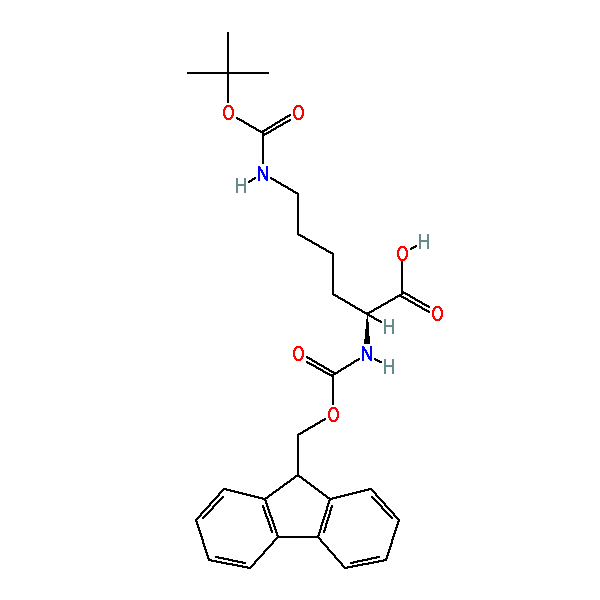
Fmoc-Lys(Boc)-OH
Nα-Fmoc-Nε-Boc-L-lysine, CAS: 71989-26-9, MW: 468.54, Formula: C26H32N2O6
(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid
Fmoc-Lys(Boc)-OH is used routinely in solid phase peptide synthesis involving Rink and Wang resins in coupling to primary and secondary amines. The side chain epsilon amine is blocked by the Boc (t-butyloxycarboyl) group which is labile to TFA (trifluoroacetic acid) cocktail during the cleavage process but inert to the repeated deprotection process with 25% piperidine in dimethlyformamide(DMF) of the Fmoc alpha amine during the synthesis of the peptide. The Boc group is removed when the peptide is cleaved from Wang resin, but is not removed under milder cleavage conditions used with trityl chloride resins, Sieber resin and PAL resin. There should be clear solubility of the material 1mM in 20 DMF. Selective removal of other side chain blocking groups available as well (Mtt-1.2.) (Aloc-3).
Storage should be under refrigerated conditions until time of use.
1. A. Aletras, et al. Int. J. Peptide Protein Res., 1995, 15, 488-96.
2. Based on D. Li and D.L. Elbert, J. Pept. Res., 2002, 60, 300-3.
3. Doi, T.; Numajiri, Y.; Munakata, A.; Takahashi, T. Org. Lett. 2006, 8, 531-534
| Catalog Number | 41001 |
|---|---|
| CAS | 71989-26-9 |
| M.W. | 468.54 |
| Formula | C26H32N2O6 |
| IUPAC Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid |
| Synonym | Nα-Fmoc-Nε-Boc-L-lysine |
| Also Known As |
|
| InChIKey | UMRUUWFGLGNQLI-QFIPXVFZSA-N |
| InChI | InChI=1S/C26H32N2O6/c1-26(2,3)34-24(31)27-15-9-8-14-22(23(29)30)28-25(32)33-16-21-19-12-6-4-10-17(19)18-11-5-7-13-20(18)21/h4-7,10-13,21-22H,8-9,14-16H2,1-3H3,(H,27,31)(H,28,32)(H,29,30)/t22-/m0/s1 |
| SMILES | CC(C)(C)OC(=O)NCCCC[C@@H](C(=O)O)NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 |