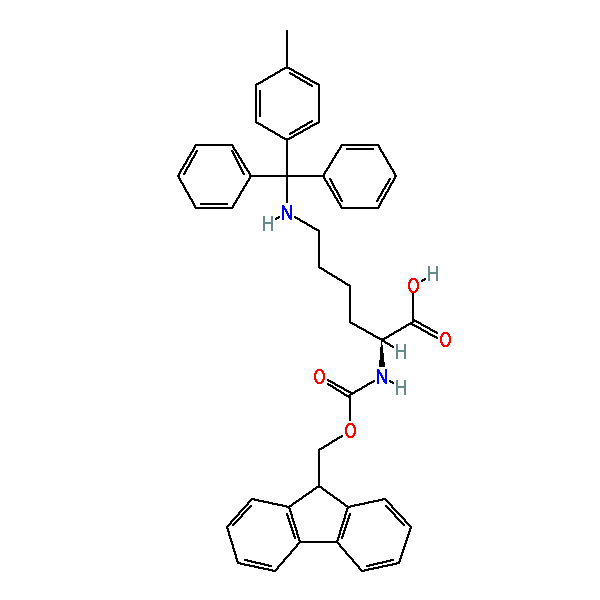
Fmoc-Lys(Mtt)-OH
N-α-Fmoc-N-ε-4-methyltrityl-L-lysine, CAS: 167393-62-6, MW: 624.77, Formula: C41H40N2O4
The side chain protecting group can be cleaved with 1%TFA in DCM (1. 2. 3.) or DCM:HFIP:TFE:TES (6.5:2:1:0.5) (4). Other scavengers such as TIS and water can be used as well depending on the sequence and other reaction conditions/side chain protection groups used before the introduction of this amino acid derivative.. This makes it an great candidate for branch chained peptide synthesis endeavors, peptides modified at the epsilon position of lysine (5. 6.) and for construction of templates for combinatorial chemistry experiments.
1. K. Barlos, et al., C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 283.
2. A. Aletras, et al. (1995) Int. J. Peptide Protein Res., 45, 488.
3. L. Bourel, et al. (2000) J. Peptide Sci., 6, 264.
4. K. Barlos, personal communication.
5. P. Hoogerhout, et al. (1999) J. Peptide Res., 54, 436.
6. C. Park & K. Burgess (2001) J. Comb. Chem., 3, 257.
| Catalog Number | 41088 |
|---|---|
| CAS | 167393-62-6 |
| M.W. | 624.77 |
| Formula | C41H40N2O4 |
| IUPAC Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-6-[[(4-methylphenyl)-diphenylmethyl]amino]hexanoic acid |
| Synonym | N-α-Fmoc-N-ε-4-methyltrityl-L-lysine |
| Also Known As |
|
| InChIKey | YPTNAIDIXCOZAJ-LHEWISCISA-N |
| InChI | InChI=1S/C41H40N2O4/c1-29-23-25-32(26-24-29)41(30-14-4-2-5-15-30,31-16-6-3-7-17-31)42-27-13-12-22-38(39(44)45)43-40(46)47-28-37-35-20-10-8-18-33(35)34-19-9-11-21-36(34)37/h2-11,14-21,23-26,37-38,42H,12-13,22,27-28H2,1H3,(H,43,46)(H,44,45)/t38-/m0/s1 |
| SMILES | CC1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)NCCCC[C@@H](C(=O)O)NC(=O)OCC4C5=CC=CC=C5C6=CC=CC=C46 |