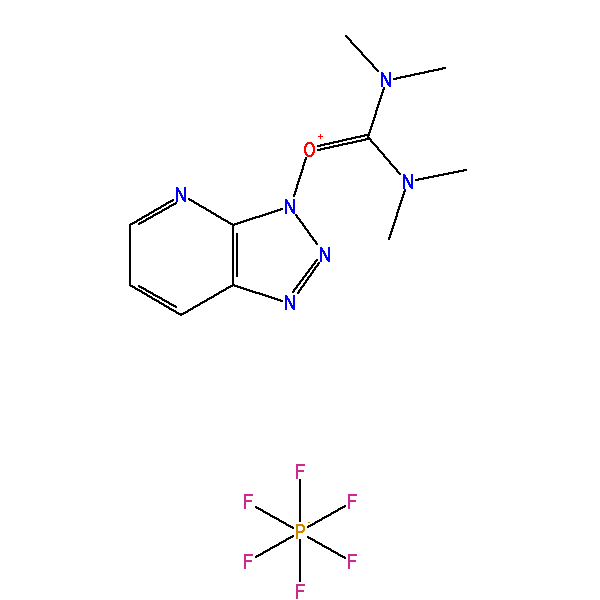
HATU
O-(7-Azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate, CAS: 148893-10-1, MW: 380.23, Formula: C10H15F6N6OP
Hazardous Material
Ground Shipment Only
HATU
O-(7-Azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
(1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate)
HATU is commonly encountered in alcohol and amine acylation reactions (i.e., ester and amide formation). Such reactions are typically performed in two distinct reaction steps: (1) reaction of a carboxylic acid with HATU to form the OAt-active ester; then (2) addition of the nucleophile (e.g., alcohol or amine) to the active ester solution to afford the acylated product.
In the first step, the carboxylate anion (formed by deprotonation by an organic base attacks HATU to form the unstable O-acyl(tetramethyl)isouronium salt. The OAt anion rapidly attacks the isouronium salt, affording the OAt-active ester and liberating a stoichiometric quantity of tetramethylurea. Addition of a nucleophile, such as an amine, to the OAt-active ester results in acylation. The solubility should be 1mM in 20mL of DMF.
Storage should be under refrigerated conditions until time of use.
1. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive J. Am. Chem. Soc., 1993, 115 (10), pp 4397–4398
| Catalog Number | 31006 |
|---|---|
| CAS | 148893-10-1 |
| M.W. | 380.23 |
| Formula | C10H15F6N6OP |
| IUPAC Name | [dimethylamino(triazolo[4,5-b]pyridin-3-yloxy)methylidene]-dimethylazanium hexafluorophosphate |
| Synonym | O-(7-Azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate |
| Also Known As |
|
| InChIKey | JNWBBCNCSMBKNE-UHFFFAOYSA-N |
| InChI | InChI=1S/C10H15N6O.F6P/c1-14(2)10(15(3)4)17-16-9-8(12-13-16)6-5-7-11-9;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1 |
| SMILES | CN(C)C(=[N+](C)C)ON1C2=C(C=CC=N2)N=N1.F[P-](F)(F)(F)(F)F |