HBTU
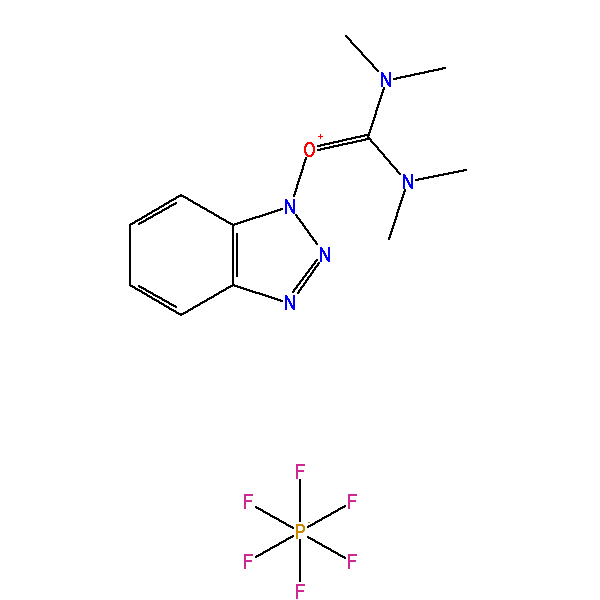
2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, CAS: 94790-37-1, MW: 379.24, Formula: C11H16F6N5OP
HBTU (2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate)
This along with TBTU are two of the most popular insitu activation reagents used in solid and solution phase peptide synthesis (1, 2.). Comparative experiments between HBTU and TBTU have shown that the counter ion has no influence on the influence of coupling rates or levels of enantiomerization (1). Coupling rates proceed smoothly and can be enhanced by the addition of HOBt (2). In addition to having high reactivity, TBTU and HBTU have shown to limit enantiomer during fragment condensation and during DMAP catalyzed esterification of arginine derivatives (3).
1. R. Knorr, et al (1989)Tetrahedron Letters, 30 1927.
2. P.A. Baybayan, et al in “ Chemistry and Biology: Proceeding of the 12th American Peptide Symposium, page 566.
3. C.G. Fields, et al (1991) Peptide Res., 95.
4. D. Ambrosius, et al. (1989)Biol. Chem. Hoppe-Seyler, 370, 217.
| Catalog Number | 31001 |
|---|---|
| CAS | 94790-37-1 |
| M.W. | 379.24 |
| Formula | C11H16F6N5OP |
| IUPAC Name | [benzotriazol-1-yloxy(dimethylamino)methylidene]-dimethylazanium hexafluorophosphate |
| Synonym | 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| Also Known As |
|
| InChIKey | UQYZFNUUOSSNKT-UHFFFAOYSA-N |
| InChI | InChI=1S/C11H16N5O.F6P/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16;1-7(2,3,4,5)6/h5-8H,1-4H3;/q+1;-1 |
| SMILES | CN(C)C(=[N+](C)C)ON1C2=CC=CC=C2N=N1.F[P-](F)(F)(F)(F)F |