PyAOP
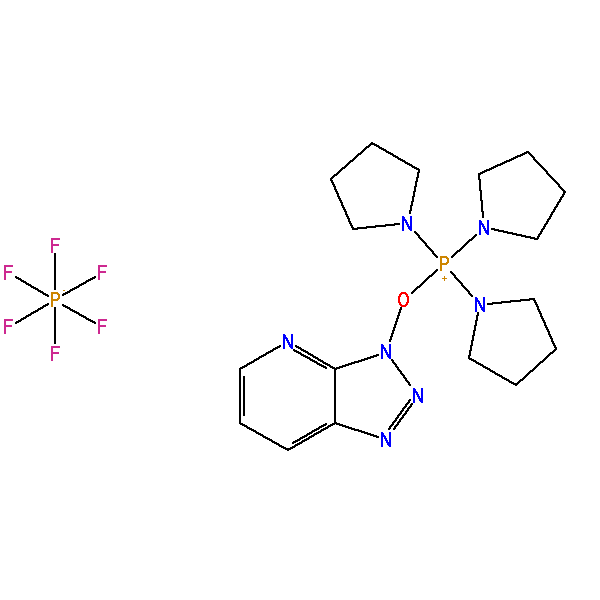
High Purity >99%, (7-Azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate, CAS: 156311-83-0, MW: 521.38, Formula: C17H27F6N7OP2
PyAOP >99% Pure (7-Azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate
The coupling agent PyAOP is a derivative of the HOAt family useful in preparing a range of difficult peptides especially amino acids of a hindered nature. An advantage of this uronium salt is that it can be used in greater excesses and does not under go detrimental side reactions at the amino terminus which blocks further assembly of a Fmoc amino acids(1.).
The pyrrolidino derivatives like PyAOP appear to be the reagent of choice relative to the piperidino compounds or those formed from trialkylamines. Further it has been determined that the various other aminium/onium salts (HOAt and HOBt) should be used for the activation of hindered species, since the latter may lead to the formation of guanidino derivatives (2.).
1. Fernando Albericio, Marta Cases, Jordi Alsina, Salvadore Triolo, Louis Carpino and Steven Kates. Tetrahedron Letters, Volume 38, Issue 27, 7 July 1997 Pages 4853-4856.
2. Fernando Albericio, Josep Bofill. Ayman El-Faham and Steven Kates, J. Org Chem. 1998; 63(26) pp 978-9683. The Journal of Organic Chemistry, 1998, 63 (26) Pages 9678-9683.
| Catalog Number | 31007 |
|---|---|
| CAS | 156311-83-0 |
| M.W. | 521.38 |
| Formula | C17H27F6N7OP2 |
| IUPAC Name | tripyrrolidin-1-yl(triazolo[4,5-b]pyridin-3-yloxy)phosphanium hexafluorophosphate |
| Synonym | (7-Azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate |
| Also Known As |
|
| InChIKey | CBZAHNDHLWAZQC-UHFFFAOYSA-N |
| InChI | InChI=1S/C17H27N7OP.F6P/c1-2-11-21(10-1)26(22-12-3-4-13-22,23-14-5-6-15-23)25-24-17-16(19-20-24)8-7-9-18-17;1-7(2,3,4,5)6/h7-9H,1-6,10-15H2;/q+1;-1 |
| SMILES | C1CCN(C1)[P+](N2CCCC2)(N3CCCC3)ON4C5=C(C=CC=N5)N=N4.F[P-](F)(F)(F)(F)F |